As announced in a press release published yesterday, HKUMed has shared its recently developed rapid test for the new coronavirus with public health laboratories around the world. But how exactly does a rapid test work? Why does it require several hours of lead time for results to return? If the test returns with a false negative result, then is the testing process at all efficacious? Professor Leo Poon, Clinical Professor of Public Health at HKU Medicine, addresses these concerns in our latest Q&A session with him.
Q1. How does a rapid test work?
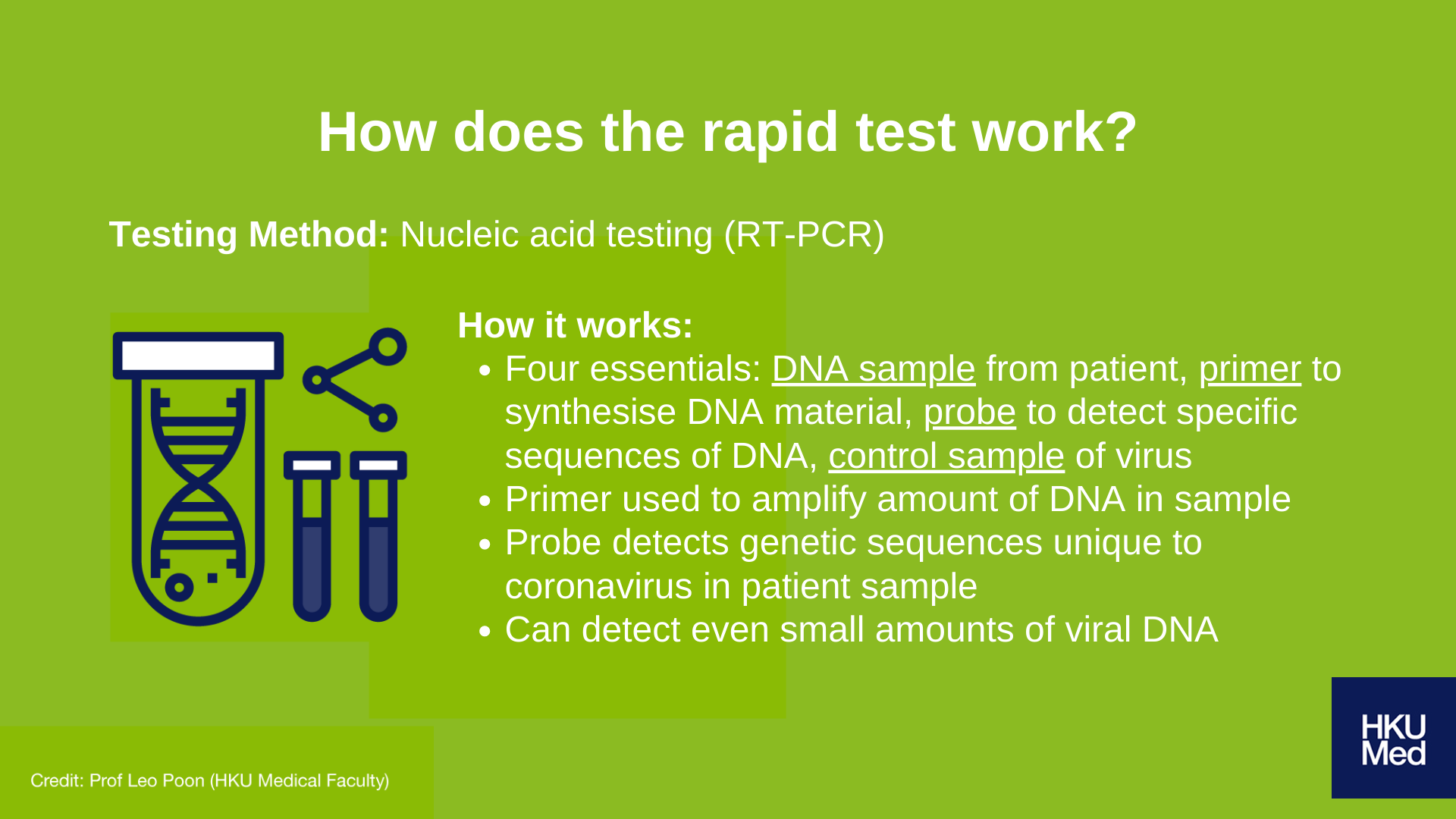
The purpose of nucleic acid testing is to ascertain the genetic code that will help us identify the virus. Based on the genetic sequence of the coronavirus that was recently released, researchers were able to design a virus-specific probe to detect the presence of virus’s unique genetic sequencing.
This process is known as RT-PCR testing, and is widely applied to the testing of various diseases such as AIDS and seasonal influenza.
Q2. Why does the rapid test take so long? Are there other slower but more accurate alternate testing methods?
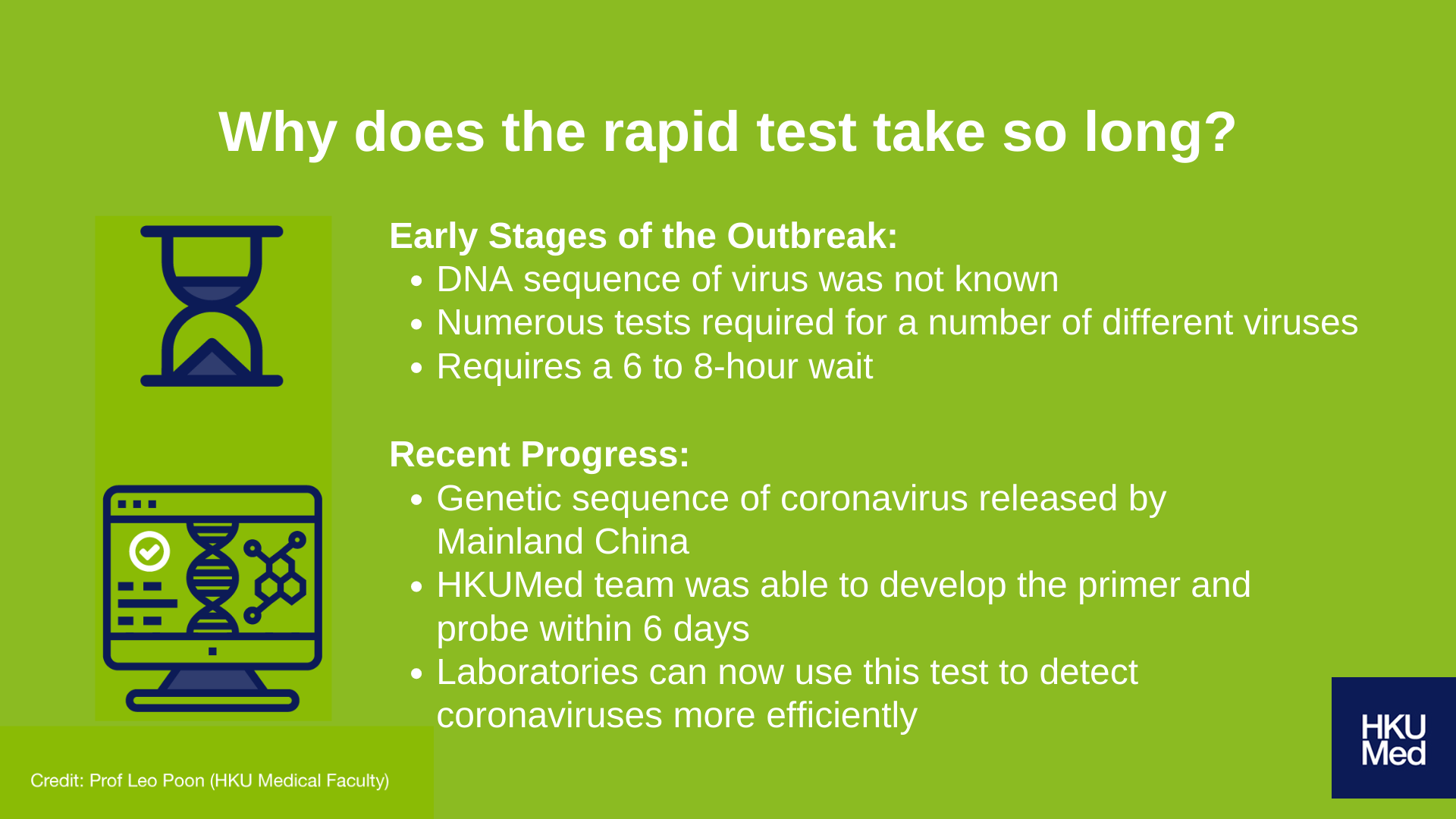
Durig the early stages of the outbreak, the virus and its DNA sequencing was not known. As a result, multiple rapid tests were required to test for a number of viruses, hence resulting in up to a 6 to 8 hour wait for results to come back.
However, with the release of the coronavirus’s genetic sequence by Mainland China, the research team at HKUMed was able to design the virus-specific probe and primer required to detect the coronavirus within six days. Public health laboratories have since been able to use this test to pinpoint the presence of the new coronavirus with much greater efficiency, subsequently leading to speedier confirmations of infected cases and identification of close contacts; this is extremely crucial in minimising community outbreaks.
In terms viable alternate tests that are more comprehensive, methods such as viral culture and detection of viral proteins are indeed theoretically possible, but they are inefficacious in the current context of disease control. For instance, viral culture must be done in a P3 laboratory, and cannot be carried out in hospitals, while the application of viral protein detection has yet to mature in clinical settings.
Q3. Earlier testing for the coronavirus with a domestic helper returned with false negative results. What is a “false negative”? Does that mean the test is not precise enough? Why are multiple tests required?

The incubation period of the novel coronavirus can be very long, while viral shedding may only occur after three to four days of infection. If the testing is carried out during a time when viral concentrations are low, the rapid test may not be able to detect the virus.
In theory, nucleic acid test is of course a quick method to test for the novel coronavirus. However, in the context of disease prevention and control measures, the timeliness and accuracy of the test becomes all the more important. In view of our current situation and its limitations, there are four factors that may affect this:
- Sampling error: samples obtained from nose and lower respiratory tract are most commonly used for nucleic acid test sampling. However, since the infection process of the novel coronavirus is not completely clear, the optimal timing of sampling is still uncertain. In addition, as the sampling process has not been standardised yet, subsequent viral isolation and diagnosis results may be affected.
- Transport restrictions of sample: the genetic material used for testing the virus can be easily degraded by environmental factors. As such, preservation and transportation of samples may also affect testing results.
- Discrepancy in quality of the testing kit: each manufacturer of the testing kit may source from different suppliers, and as such factors such as enzyme activity and DNA primer purity may affect accuracy of testing results.
- Lack of research in large quantities of viral samples and clinical efficacy of virus testing: the novel coronavirus is an RNA virus that is susceptible to mutation. As research on the virus has just begun, scientists do not have a comprehensive understanding yet of the virus i.e. its mutation frequency and mutation hotspots. In addition, research in the clinical efficacy of virus testing is lacking, meaning it is difficult rule out the possibility of current tests being unable to detect the virus due to any potential mutations.
Due to the above limitations of nucleic acid testing, the guidelines of WHO currently suggests for a confirmed diagnosis of the new coronavirus only after two positive test results; this is to account for potential false positives and false negatives, and to ensure the safety of both the patients and the general public.
Q4. Given the limitations of rapid testing, are CT scans a possible alternative?

Testing for the novel coronavirus is currently done through nucleic acid testing as mentioned. With the possibility of false negative results however, Mainland scholars are now proposing CT scans as the primary method of diagnosis instead, meaning those with negative results for the virus from nucleic acid testing but positive results from a CT Scan will also need to be treated in isolation.
However, it is important to note a doctor’s process to diagnosis takes into consideration an array of factors including medical history, signs and symptoms, testing results, and conclusions drawn from clinical observation. While imaging technologies are valuable in diagnosing and identifying presence of pathogens, there are risks of other viral pneumonias or non-infectious diseases being misidentified as the new coronavirus. As such, nucleic acid testing and CT scans are complementary of one another, providing medical professionals with a more accurate diagnosis of the new coronavirus.
At the time of writing, public health laboratories in over 40 countries have utilised the rapid test for coronavirus developed by HKUMed. Laboratories worldwide may also obtain relevant research findings and materials free-of-charge from the World Health Organisation (WHO) or HKUMed. In addition, the HKUMed WHO Collaborating Centre for Infectious Disease Epidemiology and Control processes samples from around the world, and conduct tests as necessary to facilitate efforts against the current outbreak.
Since the SARS outbreak in 2003, research teams at HKUMed’s School of Public Health have worked tirelessly to further our understanding on infectious diseases. With years of experience in handling numerous public health emergencies over the years, the School has remained at the frontlines of fighting disease outbreaks both locally and globally. Through making our research findings widely available, we hope to face this outbreak together with our peers and fellow communities around the world.
Download & Share Infographics