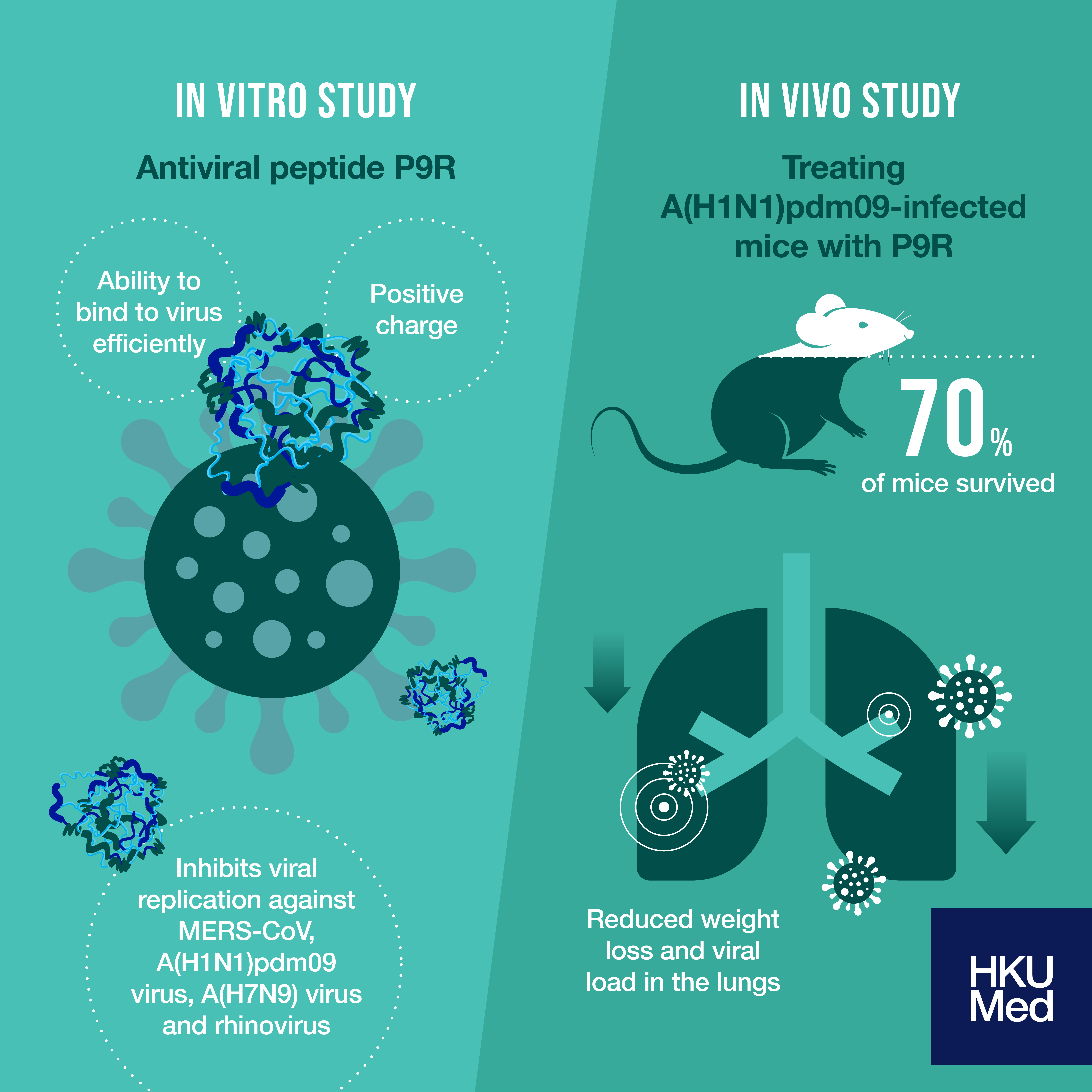
Experts from HKUMed reported the antiviral activity of peptide P9R against a number of viruses such as SARS-CoV-2, MERS-CoV, SARS-CoV, A(H1N1)pdm09, A(H7N9) virus and rhinovirus. P9R is a positively charged peptide that binds to viruses and inhibits virus-host endosomal acidification – a vital process for viral replication and infection. It is thought that the antiviral peptide works only when both processes are present. The antiviral activity of P9R was compared with PA1 and P9RS. Both PA1 and P9RS are antiviral peptides: the former only binds to viruses and the latter only inhibits endosomal acidification. Study results were published in Nature Communications.
In vitro study:
- Adding P9R to cells infected with SARS-CoV-2 inhibited viral replication. Stronger inhibition of viral replication was observed against MERS-CoV, A(H1N1)pdm09 virus, A(H7N9) virus and rhinovirus in P9R than in P9 (P9R was derived from P9 by switching weakly positively charged amino acids for more positive ones).
- Increasing the positive charge of P9R improved antiviral activity. However, P9RS had the same positive charge as P9R, but was unable to inhibit viral replication as the peptide could not bind to viruses.
- Conversely, PA1 could bind to viruses efficiently; however, the peptide was unable to inhibit endosomal acidification as it was only weakly positively charged.
- A(H1N1)pdm09 was less likely to develop resistance against P9R than against zanamivir (an antiviral medication used for influenza).
In vivo study:
- A total of 70% of mice infected with A(H1N1)pdm09 survived when treated with P9R. This number was 80% in zanamivir-treated mice.
- P9R reduced weight loss and viral load in the lungs of mice compared with phosphate-buffered saline used in the control group.
Antiviral peptides such as P9R that can bind to a variety of viruses, are able to inhibit endosomal acidification and have a low risk of inducing viral resistance; their potential antiviral activity warrants further investigation.
To read the original article published in Nature Communications, click here.