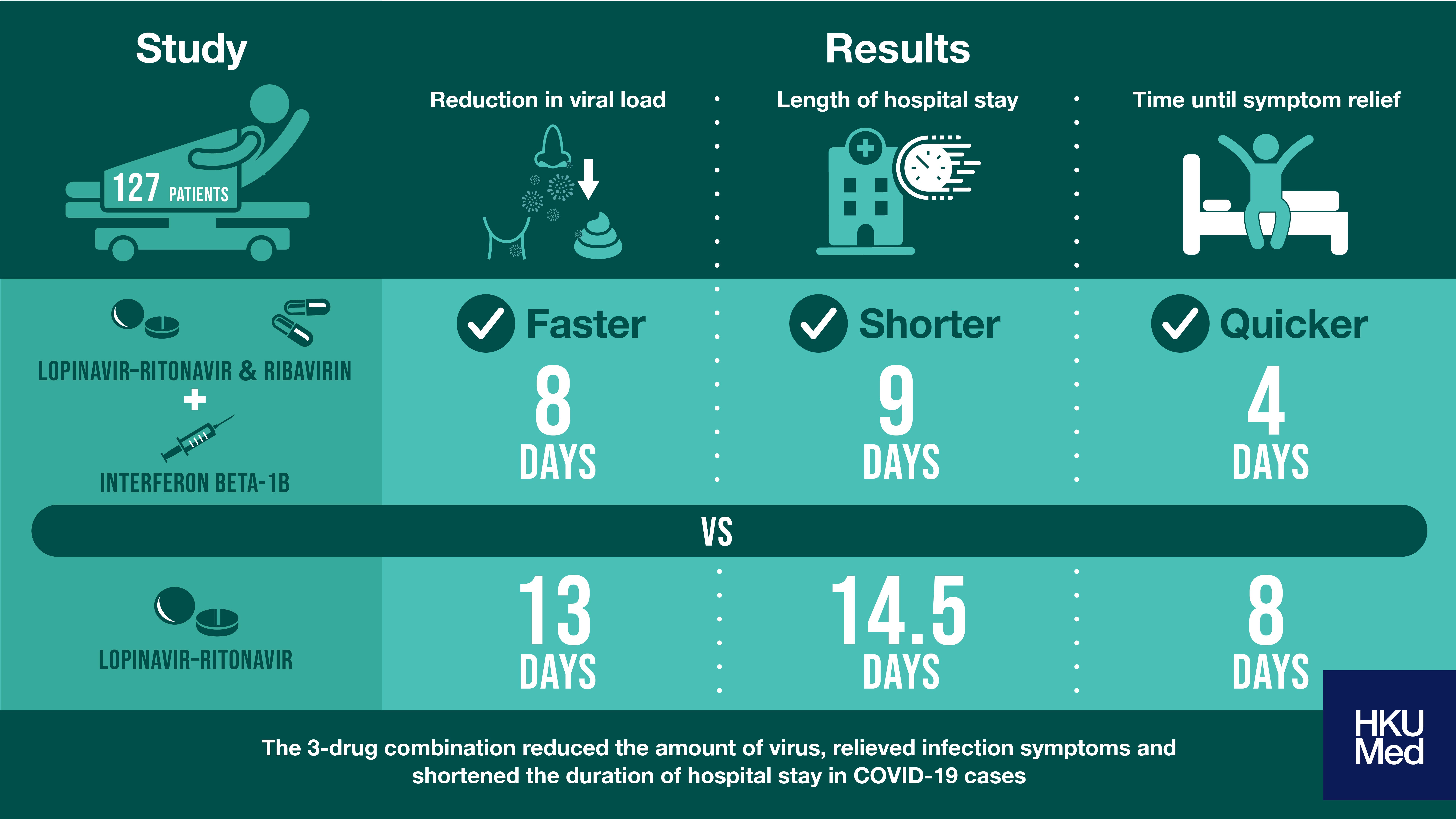
A team of experts from HKUMed was the first to conduct a controlled study to compare the use a three-drug (interferon beta-1b, lopinavir–ritonavir and ribavirin) antiviral combination with lopinavir–ritonavir (control group) in patients infected with SARS-CoV-2. In total, 127 patients participated in the study. The median age was 52 years; 54% were men; and 40% had other medical conditions. The median time from symptoms first appearing to hospitalization was 5 days. Study results were published in The Lancet, one of the world’s leading scientific journals.
Key takeaways from the study:
- The three-drug antiviral combination was effective at reducing the amount of virus in the nose, throat and stool, 8 days after starting the treatment.
- The three-drug combination also relieved all symptoms within 4 days, shorter than the 8 days recorded in the control group.
- Patients treated with the three-drug combination had a shorter stay in the hospital than the control group (9 days versus 14.5 days).
- Common side effects of this three-drug antiviral treatment were diarrhoea, fever, increased liver enzymes and vomiting, which lasted for up to 3 days.
This study showed that treating patients early with this three-drug combination was safe and effective. The antiviral combination reduced the amount of virus (viral load), relieved infection symptoms and shortened the duration of hospital stay in COVID-19 cases with mild-to-moderate symptoms. A patient is less infectious if the virus is quickly cleared in the nose, throat and stool.
To read the original article published in The Lancet, click here.